December 23, 2020
Vaccines are Here… But Keep Your Social Distance

Less than one year ago, on December 31st, 2019, an online post by the Wuhan Municipal Health Commission regarding cases of “pneumonia of unknown cause” caught the attention of the World Health Organization’s staff in China. The cause was eventually identified to be a SARS-type virus, SARS-CoV-2, which causes COVID-19. For all of us, this New Year’s Eve will feel very different than the one we celebrated in 2019.
COVID-19 infected 75.1 million worldwide and caused 1.7 million deaths as of December 20th (WHO); in the U.S., CDC reports 17.6 million cases and 315,000 deaths. However, with incredible scientific achievement — fueled by billions of federal dollars — vaccines for coronavirus were developed at unprecedented speed. The first vaccine in the U.S., a Pfizer-BioNTech product, was given Emergency Use Authorization (EUA) by the Food and Drug Administration on December 11th, and the second, by Moderna, followed on December 18th.
Step back and recognize this is a momentous week in the history of public health, to have a 94% plus vaccine, safe and effective…approved within 11 months of a novel pathogen hitting our shores… we’re at the beginning of the end of this terrible pandemic.
HHS Secretary Azar, December 16th
Success at vaccine development makes the next challenge vaccine distribution and administration. The federal government is managing a centralized system to order, track, and distribute vaccines; states, localities, Tribes and territories (SLTTs) will order vaccines through CDC within their allocations (see Chart I for a depiction of the process). The Administration has been clear that the distribution of vaccines is the responsibility of SLTTs, with the support of medical guidance from CDC, critical infrastructure advice from the Department of Homeland Security (DHS), and logistical and planning help from Operation Warp Speed. As DHS stated in a vaccine advisory memo on December 16th, “State, local, tribal, and territorial governments are responsible for implementing and executing response activities in their communities, while the Federal Government is in a supporting role. Officials should use their own judgment in making decisions regarding resource allocation and other public health measures.”
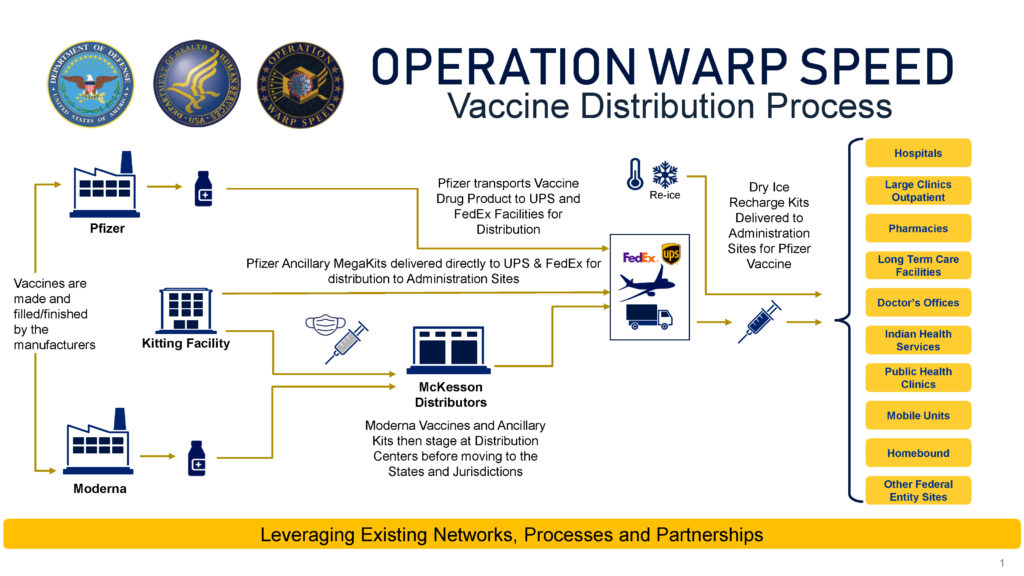

Chart I. Source: Operation Warp Speed.
Highlighting the states’ role, General Perna, the COO of Operation Warp Speed, on December 16th noted “powerful collaboration” with pharmacies (CVS and Walgreens) and CDC, but “most importantly enabling the governor’s plans to expand vaccination throughout their states.”
Governors are extremely concerned about distribution costs, given the winter spike in COVID-19 cases, the size, scale and urgency of the project, and their strained budgets. The December COVID supplemental includes the first federal funding specific to vaccine distribution. The bill provides $8.75 billion for vaccine distribution, of which $4.5 billion goes directly to states and localities and the remainder to CDC. In addition, there is $22 billion for testing and contact tracing, $20 billion for federal procurement of vaccines and therapeutics and $3 billion for the national stockpile. The Biden-Harris COVID plan includes a marker of $25 billion for vaccine manufacturing and distribution, with the intention of providing cost-free vaccines for every American.
Distribution and administration costs are significant because of storage, dosing, scale, and administrative requirements. The first vaccines require two doses per person and cold storage. The Pfizer product must be kept between -112°F and -76°F until diluted, and the Moderna product between -13°F and 5°F. Pfizer’s two doses must be administered 21 days apart, and Moderna’s 28 days apart. Administrative requirements include patient follow-up for a minimum of two months, documentation of side effects, mandatory reporting of adverse reactions, and recordkeeping to support an eventual application to FDA for permanent approval.
Both vaccines, as well as several other candidates in the pipeline, include a new approach to vaccine development, using “messenger” RNA (mRNA). The new technology means it is even more critically important to gather and process data to understand vaccine safety and effectiveness in the inoculated population. As Operation Warp Speed cautions: “Regarding vaccination, we won’t know how long immunity lasts until we have a vaccine and more data on how well it works.”
Logistics are handled by the federal government with military support. Twenty-six states called up the National Guard to assist with vaccine distribution. Even so, challenges remain with prioritization due to low supply. For each vaccine that FDA allows on the market through the EUA process, CDC’s Advisory Committee on Immunization Practices (ACIP) makes recommendations for the use of the vaccine. On December 13th, ACIP recommended the Pfizer vaccine for use in people ages 16 and up for the prevention of COVID-19, but noted their overall guidance that “vaccination in the initial phase of the COVID-19 vaccination program (Phase 1a) should be offered to both 1) healthcare personnel and 2) residents of long-term care facilities.” Other federal planning guidance on vaccine rollout prioritization is included in CDC’s vaccine playbook, (Centers for Disease Control and Prevention (CDC) COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations) and a Department of Homeland Security Advisory Memo issued December 16th that focuses on critical infrastructure workforce.
States are not bound by this guidance, but press reports nationwide indicate that healthcare personnel have been the first vaccine recipients. Decisions for jurisdictions will become more difficult as vaccine supply increases. While there will be more doses, both from Pfizer and Moderna, there won’t yet be enough to cover phase 1a, let alone additional vulnerable populations. Each state will make different decisions regarding the elderly, people with pre-existing conditions, non-healthcare essential workers, teachers, prisoners, or COVID hotspots.
Vaccine Allocations |
Pfizer |
Moderna |
|
Doses Allocated for Distribution |
2,071,875 |
5,990,000 |
|
Doses Allocated to Hold for Second Shot |
2,071,875 |
5,948,600 |
|
Safety Stock Allocated |
156,250 |
561,400 |
| Total | 4,300,000 | 12,500,000 |
Chart II. Source: HHS Dec. 19 Data, COVID 19 Vaccine Distribution/Allocation.
The data on numbers of available vaccines and allocations has been inconsistent, but on December 19th HHS reported that the federal government will initially distribute just over two million Pfizer vaccines and close to six million Moderna vaccines, with allocations based on adult population and additional amounts for several cities. See state-by-state allocations for the Pfizer vaccine and for the Moderna vaccine.
With eight million first doses available (plus an equal number of the required second doses), the first tranche of vaccination can only cover one-third of the approximately 20 million health care workers and three million residents of long-term health facilities. States differ in their approaches for phase 1a. For example, Kentucky is focusing on people in long-term care facilities and then health-care workers; Alabama is taking the opposite approach. Hospitals need to triage their staff since they will not have enough to vaccinate everyone. University of California San Francisco hospital has 10,000 staff with direct patient care responsibilities but expects to receive only enough doses for one-third of them in the first tranche.
A further complication is that no vaccine testing was done on pregnant women or children under 16. While manufacturers are now expanding research cohorts by age, there will be significant delays in pediatric use information. Women of childbearing age make up a large proportion of the U.S. healthcare workforce. For those who are pregnant, there is no data on which to make vaccine decisions, a concern that the FDA Vaccine Advisory Committee noted in its deliberations.
Operation Warp Speed officials, Secretary Azar, General Perna, and Chief Advisor Slaoui, held a press conference on December 16th to address distribution, supply, and the vaccine pipeline. From now until March 31st, Secretary Azar expects a total of 100 million Pfizer doses and 100 million Moderna doses, and in the second quarter, 100 million additional Moderna doses (in total covering 150 million people); he is “very optimistic” about obtaining more Pfizer vaccines in the second quarter. Next in the pipeline, Secretary Azar expects a single-dose Johnson & Johnson/Jansen vaccine (100 million people). AstraZeneca is contracted for 300 million doses (covering 150 million). Two additional vaccines, manufactured by Sanofi and Novavax, will follow. In total, if all the vaccines in this pipeline achieve EUA, the federal government will have 900 million doses of vaccine under contract, with options for more. The demand for tracking and monitoring technologies to support administration of these vaccines will be considerable.
While optimistic, Secretary Azar cautioned that we need patience: “Even with the emergence of these new tools, we are not at the finish line yet, especially as we approach the holidays. And in the coming months, we’ve all got to continue taking steps to keep ourselves, our loved ones, and our communities safe.”