March 21, 2022
FY22 Omnibus Health-focused Funding and Policy


In his FY22 budget request, President Biden proposed major investments in public health, including disease prevention and treatment, surveillance and data analysis, and research and development. The primary appropriations vehicle for health-related funding is the Labor-Health and Human Services (HHS)-Education bill, which includes funding for the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), the public health emergency fund, the Biomedical Advanced Research and Development Authority (BARDA), the Strategic National Stockpile and the Centers for Medicare and Medicaid Services (CMS).
The Labor-HHS-Education bill approved by the House in July included a massive 28% increase over FY21 funding. As part of the negotiation process with the Senate, lower top-line numbers were set, necessitating cuts to administration and House-proposed levels. However, even with top-line constraints, Congress provided significant public health investments in the omnibus, including $1 billion for a new experimental health research program. Research, preparedness, public health infrastructure, and telehealth advances are highlighted below.
Now, I would like to add a word about another investment this bill makes, one that I expect will pay dividends for hope, healing, and for our economy for generations to come. And it’s called ARPA-H — Advanced Research Projects Agency for Health. This will be a new kind of entity, an engine for innovation, a place where we’ll do high-risk, high-reward research that can drive unprecedented progress in biomedicine.
President Biden
March 16
Research and Development
The omnibus launched ARPA-H with $1 billion in funding available through FY24. President Biden’s FY22 budget requested $6.5 billion. The goal of ARPA-H is to accelerate the pace of breakthroughs in medicine through a model similar to that of the Department of Defense’s Defense Advanced Research Projects Agency (DARPA) via grants, contracts, and cooperative agreements. ARPA-H will operate on time-limited research projects with quantifiable goals, with the expectation of a “not afraid to fail” mentality in order to encourage risk-taking. Initial research will focus on cancer, diabetes, and Alzheimer’s disease. The legislation requires the President to appoint a director for ARPA-H; after that, expect administrative set up, hiring of project managers, and appointing an external advisory board to take several months.
NIH is appropriated close to $45 billion, an increase of 5.3% over FY21, with targeted increases of $75 million for research related to opioids, $105 million to address health disparities, $40 million for cybersecurity, $60 million for the neurotechnology-focused BRAIN initiative; and $37.5 million to examine the effects of COVID-19. Within NIH, the National Cancer Institute received $6.7 billion, an increase of $354 million from FY21. The total includes a $150 million increase for grants, in particular those focusing on breakthrough research in genomics, computational science, immunotherapy and bioengineering. The omnibus also includes $300 million, an increase of $13 million over FY21, for pandemic influenza vaccines.
Regarding AI and “Big Data,” Congress notes concerns about the balance of safeguarding health data and using it to its maximum potential. The omnibus directs HHS to streamline and centralize controlled access mechanisms to preserve protection of data, to use best practices of data repositories, and to make data more accessible to researchers.
Preparedness and Response Capabilities, Supply Chain
The bill includes $2.8 billion for the Assistant Secretary for Preparedness and Response (ASPR), an increase of $325 million over last year’s base funding. Within that, BARDA receives $745 million, an increase of $148 million over FY21 to support development and production of novel vaccine, therapeutic, and diagnostics, including advanced manufacturing of vaccines and therapeutics.
The Strategic National Stockpile (SNS) is funded at $845 million, $140 million over FY21, to enable keeping an inventory of supplies and setting up a modern distribution model to ensure readiness for a future pandemic. The omnibus includes language requesting that the administration “re-envision” the SNS “for procurement that meets healthcare and national security needs.”
Specifically, Congress directs that re-envisioning the SNS requires: real-time inventory transparency; data and analytics; elasticity to readily scale responses; modeling and simulation for planning; and supply chain risk management. A progress report is due in 60 days.
CDC Public Health Infrastructure and Data Modernization
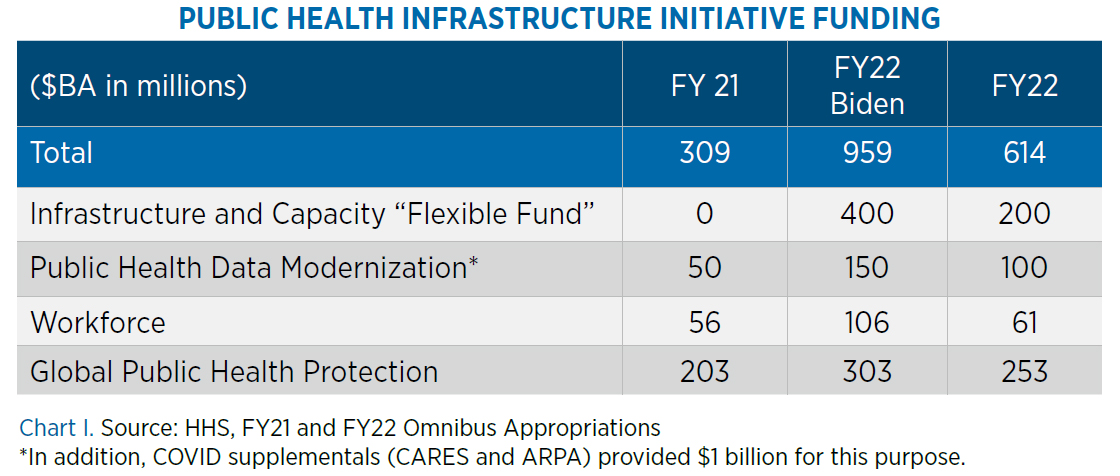
The omnibus provides $8.5 billion for CDC, a 7% increase over FY21 but less than the $9.6 billion request. The funding includes $7.5 billion in budget authority and $903 million in transferred funding, with increases focused on a multi-year effort to modernize public health data surveillance and analytics in order to provide early warning and global forecasting capabilities.
CDC funding includes: 1) $100 million for public health data modernization, to support “a modern, high-speed, networked public health infrastructure;” 2) $410 million for surveillance, epidemiology and informatics; 3) $61 million for public health workforce; 4) $253 million for global public health protection; and 5) $200 million for public health infrastructure and capacity, described as “a new funding line to provide a stable source of resources that is not segmented by disease, condition, or activity.” Congress directs that at least 70% of these funds are to be awarded to health departments. Chart I on page 7 includes a summary of Public Health Infrastructure initiative funding.

Telehealth Policy
The COVID-19 Public Health Emergency (PHE) declaration included maximum “flexibilities” for Medicare. The Biden administration extended those flexibilities as Congress debated and drafted telehealth legislation. The omnibus included an extension of the telehealth flexibilities allowed in the Medicare program during the PHE, including a waiver of Medicare’s geographic and originating site restrictions which require a patient to live in a rural area and be physically in a doctor’s office or clinic to use telehealth services, expansion of the types of health care providers or services covered, and allowing use of audio-only telehealth services.
In a “kick-the-can” action, the flexibilities were extended for 151 days after the end of the final COVID-19 Public Health Emergency (PHE) declaration but were not made permanent, despite much support in Congress and the administration. It all comes down to money: CBO estimates the cost of the 151-day extension as $663 million Permanent expansion would cost billions. Congressional negotiators have argued that use of telehealth can actually provide cost savings, but thus far telehealth expansion has been scored as a cost rather than a savings.
Also in the omnibus, Congress directs the Secretary of HHS to evaluate and report on telehealth usage during the COVID-19 PHE and its lasting impact on patient care. Further, the Centers for Medicare and Medicaid Services (CMS) is required to review audio-only services delivered during the COVID-19 PHE and to report on best practices for using telehealth and remote patient monitoring in the homeless population.
In addition to its strong growth in Medicare and in state-run Medicaid programs, telehealth continues to be a large factor in the areas in which the federal government directly provides healthcare, such as for care for veterans, military medicine, and the Indian Health Service.
Advances in Health Require Tech
Health technologies, from advanced manufacturing in BARDA programs to the compute power needed for forecasting and modeling outbreaks at CDC, are crucial to advancements in public health. Mining data and designing studies to evaluate outcomes and disparities in patient populations is a major administration priority with significant policy and funding implications. Telehealth and associated technologies for remote monitoring allowed medical teams to reach patients during the pandemic, to monitor chronic conditions remotely, and to make unprecedented inroads in mental health treatment.
The FY22 omnibus funding reflects the bipartisan priorities to advance medical care in the United States, while facing the harsh statistics that COVID-19 reduced life expectancy in the U.S. to a greater degree than it did in peer countries. With legislators and the Biden Administration seeking to reverse that trend, expect robust funding in President Biden’s FY23 budget that expand approved FY22 health-related funding increases with an eye toward transformative, tech-dependent health advances.